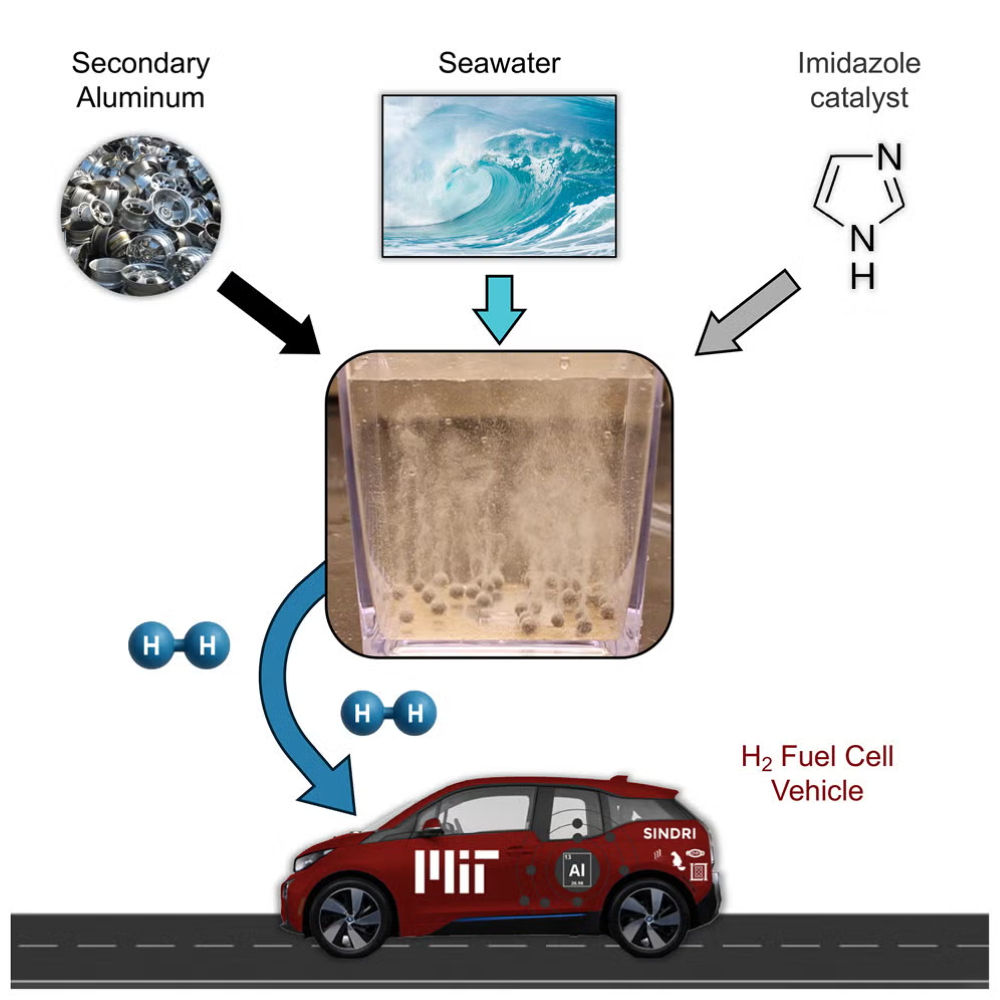
MIT's Sustainable Hydrogen Breakthrough with Recycled Aluminium and Seawater
Key Ideas
- MIT researchers develop a cost-effective method using recycled aluminium and seawater to produce low-emission green hydrogen.
- The process emits just 1.45 kg CO₂ per kg of hydrogen, with potential profits from the by-product boehmite used in industrial applications.
- A prototype hydrogen reactor was demonstrated, offering safe, solid-aluminium pellet transportation and potential for off-grid applications.
- The innovative approach leverages aluminium's abundance, energy density, and sustainability benefits to transform scrap metal and seawater into clean energy.
Researchers at MIT have unveiled a groundbreaking method to produce sustainable green hydrogen using recycled aluminium and seawater. Unlike conventional methods like steam methane reforming, which emit significant CO₂, this innovative approach harnesses the aluminium-water reaction to generate hydrogen, heat, and aluminium oxyhydroxide. MIT overcame the challenge of the oxide layer on aluminium by coating recycled pellets with a gallium-indium alloy. The process emits only 1.45 kg CO₂ per kg of hydrogen, nearing the cleanest electrolysis systems. It also produces boehmite, enhancing profitability. A prototype hydrogen reactor was showcased, highlighting the safety and logistical advantages of solid aluminium pellet transportation. This method, drawing on seawater and aluminium, shows promise for off-grid applications. By using recycled aluminium and innovative techniques, the research team envisions a scalable, low-emission pathway for hydrogen production. The system's potential for transportation, remote energy, and marine uses could revolutionize clean energy deployment in areas with limited infrastructure.