Revolutionizing Green Hydrogen Production: Innovative Catalyst for Water Oxidation
Key Ideas
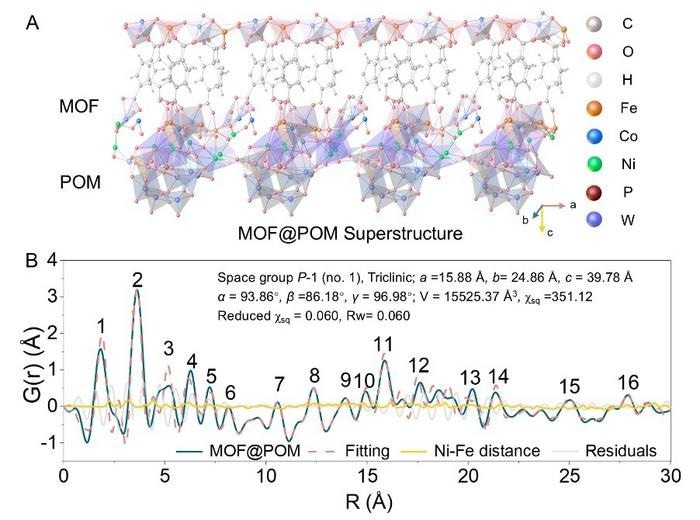
- Development of a stable and efficient catalyst comprising cobalt-iron metal-organic frameworks (CoFe-MOF) on nickel-bridged polyoxometalates (POMs) for water oxidation.
- The catalyst showed high performance in alkaline electrolytes, achieving a current density of 10 mA/cm² with only 178 mV overpotential and surpassing conventional catalysts.
- Long-term stability tests demonstrated the catalyst's robustness, operating for over 5,140 hours at 2 A/cm² with minimal voltage decay, exceeding industrial targets.
- The research paves the way for next-generation electrocatalysts, offering potential for industrial-scale, high-current, low-energy alkaline water electrolysis.
A research team led by Prof. Ya Yan from the Shanghai Institute of Ceramics developed a breakthrough catalyst for water oxidation, vital for green hydrogen production. The novel catalyst, a MOF@POM superstructure, combines cobalt-iron metal-organic frameworks with nickel-bridged polyoxometalates to achieve high catalytic activity and durability under industrial-level current densities. In electrolysis tests, the catalyst exhibited superior performance, requiring minimal overpotential and surpassing existing transition metal-based catalysts. Notably, the system showed exceptional stability, operating continuously for over 5,000 hours at room temperature. This research, published in Science, sets a new standard for water oxidation catalysts, offering a design blueprint for advanced electrocatalysts and opening avenues for large-scale, low-energy alkaline water electrolysis.