Optimizing Interfacial Water for Advanced Hydrogen Electrocatalysis
Key Ideas
- Research focuses on regulating interfacial water structures for efficient hydrogen electrocatalysis.
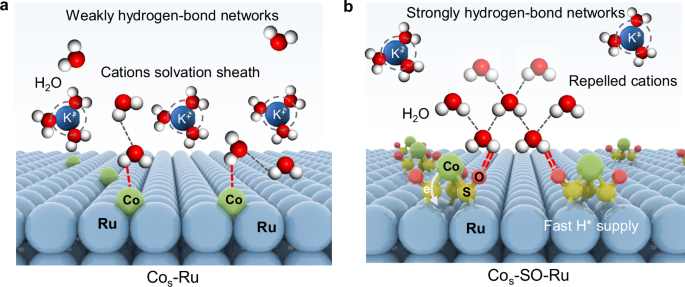
- Introduction of cobalt and ruthenium atomic pairs (Cos-SO-Ru) enhances HOR/HER activity and durability.
- Organic structures synergized with metallic active sites mimic natural metalloproteins for improved catalytic performance.
- The study provides insights into the crucial role of interfacial water in electrocatalysis and offers general principles applicable to other catalytic processes.
The article discusses the significance of optimizing interfacial water structures for advanced hydrogen electrocatalysis. By anchoring single cobalt atoms on ruthenium nanoclusters via sulfo-oxygen bridges, cobalt and ruthenium atomic pairs (Cos-SO-Ru) are created to enhance the hydrogen evolution and oxidation reactions. This configuration improves HOR/HER activity and durability compared to Co single-atom doped Ru clusters. Inspired by natural metalloproteins, the study aims to mimic enzyme systems for efficient proton transfer through organic structures synergized with metallic active sites. The research results reveal that the interfacial water is effectively regulated by the created hydrogen-bond network, facilitating H* transfer and supply. The work offers insights into the crucial roles of interfacial water in electrocatalysis and provides fundamental principles that can be applied to other catalytic processes. Structural characterization of the catalysts demonstrates the successful formation of porous carbon-supported ruthenium nanoclusters with single cobalt atoms, showcasing the potential of this approach for enhancing hydrogen electrocatalysis.
Topics
Power
Nanotechnology
Catalysis
Electrochemical
Metallic Structures
Enzyme-inspired
Atomic Pairs
Interface Modulation
Cobalt-ruthenium Clusters
Latest News