Decoupling Hydrogen Oxidation Reaction: Towards Efficient Tandem Electrocatalysis
Key Ideas
- Proposed a tandem electrocatalysis concept to enhance hydrogen oxidation reaction kinetics.
- Developed a highly efficient tandem HOR electrocatalyst, Pt1-Ru/C, with ultralow platinum group metal content.
- Experimental and theoretical evidence supports the decoupling of the HOR process and hydrogen spillover from Ru to Pt sites on the catalyst.
- The Pt1-Ru/C anode catalyst exhibited a peak power density of 1.91 W/cm² and high anodic mass activity of 23.12 A/mgPGM in PEMFCs.
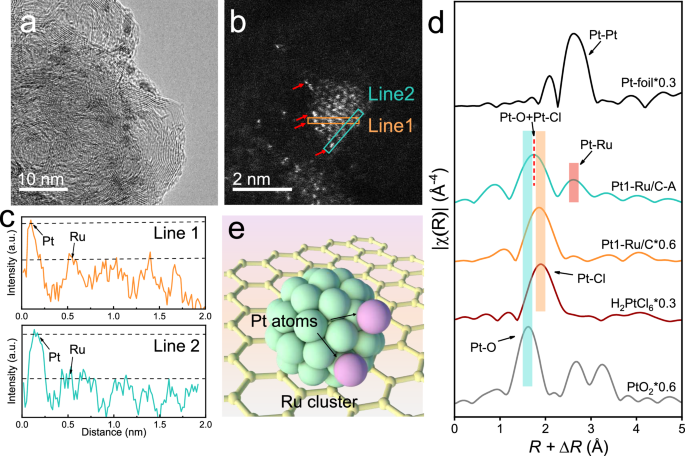
Proton exchange membrane fuel cells (PEMFCs) utilize hydrogen for electricity production, but the high cost of platinum catalysts is a key obstacle. This article proposes a novel tandem electrocatalysis approach to improve the efficiency of the hydrogen oxidation reaction (HOR). By developing the Pt1-Ru/C electrocatalyst, consisting of ruthenium nanoclusters decorated with platinum single atoms, significant advancements in reducing platinum group metal content were achieved. Experimental results confirmed the decoupling of the HOR process and showcased hydrogen spillover from ruthenium to platinum sites. Structural characterizations revealed the unique catalyst structure, with Pt single atoms anchored on Ru nanoclusters. The Pt1-Ru/C catalyst demonstrated remarkable performance in PEMFCs with a peak power density of 1.91 W/cm² and high anodic mass activity. The study provides insights into enhancing fuel cell efficiency by utilizing innovative catalyst designs and leveraging hydrogen spillover mechanisms.
Topics
Fuel Cells
Catalysts
Electrocatalysis
Energy Conversion
Platinum Group Metals
Nanoclusters
Hydrogen Spillover
Structural Characterization
Latest News